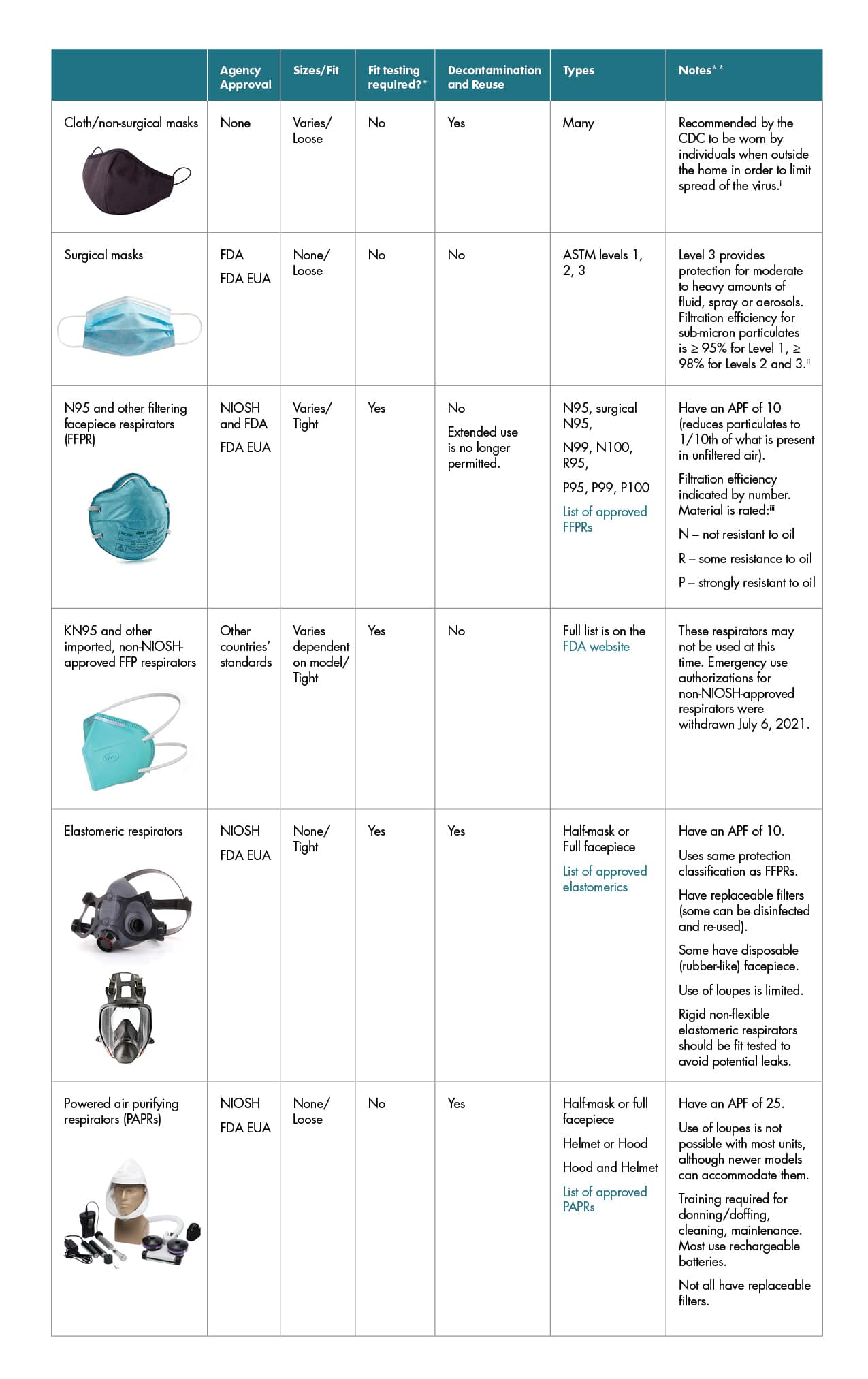
Cloth/non-surgical masks
- Agency Approval: None
- Sizes/Fit: Varies/Loose
- Fit testing required?*: No
- Decontamination and Reuse: Yes
- Types: Many
- Notes**: Recommended by the CDC to be worn by individuals when outside the home in order to limit the spread of the virus.i
Surgical masks
- Agency Approval: U.S. Food and Drug Administration and U.S. Food and Drug Administration Emergency use authorization
- Sizes/Fit: None/Loose
- Fit testing required?*: No
- Decontamination and Reuse: No
- Types: American Society for Testing and Materials levels 1, 2, 3
- Notes**: Level 3 provides protection for moderate to heavy amounts of fluid, spray, or aerosols. Filtration efficiency for sub-micron particulates is ≥ 95% for Level 1, ≥ 98% for Levels 2 and 3.ii
N95 and other filtering facepiece respirators (FFPR)
- Agency Approval: CDC National Institute of Occupational Safety and Health and U.S. Food and Drug Administration
- Sizes/Fit: Varies/Tight
- Fit testing required?*: Yes
- Decontamination and Reuse: No. Extended use is no longer permitted.
- Types: N95, surgical N95; N99, N100, R95; P95, P99, P100. For the list of approved FFPRs, visit the CDC website.
- Notes**: Have an Assigned Protection Factor of 10 (reduces particulates to 1/10th of what is present in unfiltered air). Filtration efficiency is indicated by a number. Material is rated: iii; N – not resistant to oil; R – some resistance to oil; P – strongly resistant to oil
KN95 and other imported, non-NIOSH-approved FFP respirators
- Agency Approval: Other countries’ standards
- Sizes/Fit: Varies dependent on model/ Tight
- Fit testing required?*: Yes
- Decontamination and Reuse: No.
- Types: Read the full list on the FDA website.
- Notes**: These respirators may not be used at this time. Emergency use authorizations for non-NIOSH-approved respirators were withdrawn on July 6, 2021.
Elastomeric respirators
- Agency Approval: CDC National Institute of Occupational Safety and Health and U.S. Food and Drug Administration Emergency use authorization
- Sizes/Fit: None/ Tight
- Fit testing required?*: Yes
- Decontamination and Reuse: Yes
- Types: Half-mask or Full facepiece. Read the list of approved elastomeric on the CDC website.
- Notes**: Have an Assigned Protection Factor of 10; Uses same protection classification as FFPRs; Have replaceable filters (some can be disinfected and re-used); Some have disposable (rubber-like) facepiece; Use of loupes is limited; Rigid non-flexible elastomeric respirators should be fit tested to avoid potential leaks.
Powered air-purifying respirators (PAPRs)
- Agency Approval: CDC National Institute of Occupational Safety and Health and U.S. Food and Drug Administration Emergency use authorization
- Sizes/Fit: None/ Loose
- Fit testing required?*: No
- Decontamination and Reuse: Yes
- Types: Half-mask or Full facepiece. Helmet or Hood; Hood and Helmet. Read the full list of approved PAPRs on the CDC website.
- Notes**: Have an Assigned Protection Factor of 25; Use of loupes is not possible with most units, although newer models can accommodate them: Training required for donning/doffing, cleaning, maintenance; Most use rechargeable batteries; Not all have replaceable filters.
Footnotes:
* An initial fit testing and medical evaluation should be conducted for each employee. More information can be found in the sample Respiratory Protection Program resource.
** Use of loupes and headlights should be determined on a case-by-case basis.
i N95 Respirators, Surgical Masks, Face Masks and Barrier Face Coverings, U.S. Food and Drug Administration resource on N95 Respirators, Surgical Masks, Face Maks, and Barrier Face Coverings.
ii Choose the Right Mask, Cardinal Health resource on choosing the right mask.
iii NIOSH-Approved Filtering Facepiece Respirators resource on NIOSH-Approved Particulate Filtering Facepiece Respirators accessed July 17, 2020
To access our latest resources, visit our Resource Library.